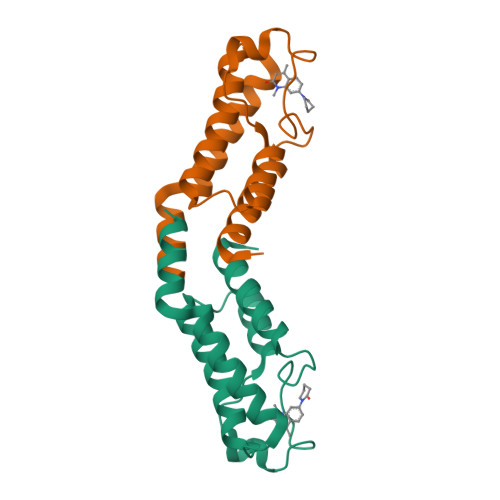
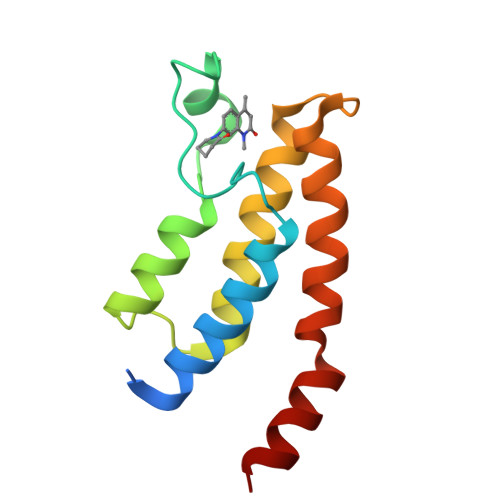
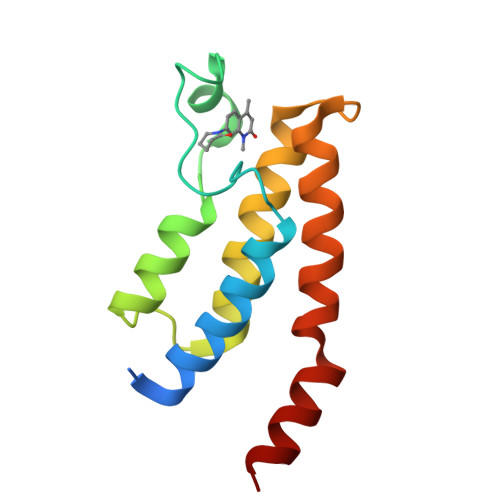
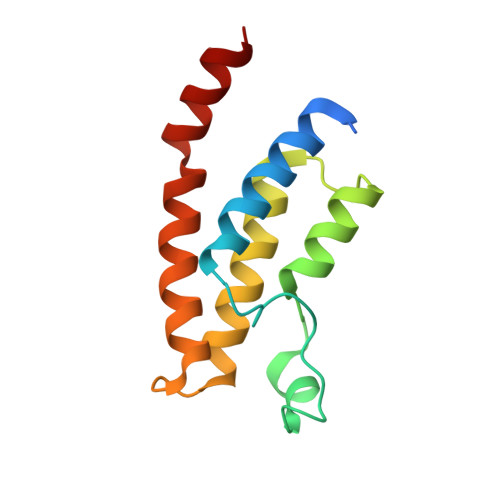
LP99: Discovery and Synthesis of the First Selective BRD7/9 Bromodomain Inhibitor.
Clark, P.G., Vieira, L.C., Tallant, C., Fedorov, O., Singleton, D.C., Rogers, C.M., Monteiro, O.P., Bennett, J.M., Baronio, R., Muller, S., Daniels, D.L., Mendez, J., Knapp, S., Brennan, P.E., Dixon, D.J.(2015) Angew Chem Int Ed Engl 54: 6217-6221
- PubMed: 25864491
- DOI: https://doi.org/10.1002/anie.201501394
- Primary Citation of Related Structures:
4Z6H, 4Z6I - PubMed Abstract:
The bromodomain-containing proteins BRD9 and BRD7 are part of the human SWI/SNF chromatin-remodeling complexes BAF and PBAF. To date, no selective inhibitor for BRD7/9 has been reported despite its potential value as a biological tool or as a lead for future therapeutics. The quinolone-fused lactam LP99 is now reported as the first potent and selective inhibitor of the BRD7 and BRD9 bromodomains. Development of LP99 from a fragment hit was expedited through balancing structure-based inhibitor design and biophysical characterization against tractable chemical synthesis: Complexity-building nitro-Mannich/lactamization cascade processes allowed for early structure-activity relationship studies whereas an enantioselective organocatalytic nitro-Mannich reaction enabled the synthesis of the lead scaffold in enantioenriched form and on scale. This epigenetic probe was shown to inhibit the association of BRD7 and BRD9 to acetylated histones in vitro and in cells. Moreover, LP99 was used to demonstrate that BRD7/9 plays a role in regulating pro-inflammatory cytokine secretion.
Organizational Affiliation:
Department of Chemistry, Chemistry Research Laboratory, University of Oxford, Mansfield Road, Oxford, OX1 3TA (UK).